Polycystic Kidney Disease (ADPKD or PKD)
Here are some useful information that will improve your eGFR and your health.
FIRST, HERE IS MY STORY (SHORT VERSION)
I am a 44 yo male, working in a non-medical field. I first discovered that I have Polycystic Kidney Disease when I was 21 yo, when I had an urinary infection. The doctors put me on ultrasound and they told me “Congratulations, you have PKD. Most probably, around 40 years old you’ll need a new kidney“.
Time passed, I’ve been to yearly controls, asking my doctor what is new in this field. Each year, almost the same story: nothing new, take care of yourself, see you next year. Creatinine levels very slowly deteriorating, no blood in urine (except 2021, when I made an intense effort).
Unfortunately, since 2022 the renal function started to deteriorate in an accelerated pattern. So bad, that in February 2023 I was sure that in maximum 1 month I had to enter on dialysis.
Since February 2023 I started to pursue keto-diet, multiple supplements, all backed-up by medical studies.
I’ve read hundreds of medical literatures/studies on PKD/ADPKD. I want to share my findings with you, hoping that helps.
My belief: don’t lease your health only to the specialists. They do not have enough time or energy to be on top of all new development on this disease.
Good luck!
Some interesting data I could find regarding the influence of nutrition (Keto Diet especially) on the evolution of PKD. Random order.
Ketogenic dietary interventions in autosomal dominant polycystic kidney disease—a retrospective case series study: first insights into feasibility, safety and effects
Source: Clinical Kidney Journal https://academic.oup.com/ckj/article/15/6/1079/6369511
Date: 2021.09.13
Abstract
Background
Our laboratory published the first evidence that nutritional ketosis, induced by a ketogenic diet (KD) or time-restricted diet (TRD), ameliorates disease progression in polycystic kidney disease (PKD) animal models. We reasoned that, due to their frequent use for numerous health benefits, some autosomal dominant PKD (ADPKD) patients may already have had experience with ketogenic dietary interventions (KDIs). This retrospective case series study is designed to collect the first real-life observations of ADPKD patients about safety, feasibility and possible benefits of KDIs in ADPKD as part of a translational project pipeline.
Methods
Patients with ADPKD who had already used KDIs were recruited to retrospectively collect observational and medical data about beneficial or adverse effects and the feasibility and safety of KDIs in questionnaire-based interviews.
Results
A total of 131 ADPKD patients took part in this study. About 74 executed a KD and 52 a TRD for 6 months on average. A total of 86% of participants reported that KDIs had improved their overall health, 67% described improvements in ADPKD-associated health issues, 90% observed significant weight loss, 64% of participants with hypertension reported improvements in blood pressure, 66% noticed adverse effects that are frequently observed with KDIs, 22 participants reported safety concerns like hyperlipidemia, 45 participants reported slight improvements in estimated glomerular filtration rate and 92% experienced KDIs as feasible while 53% reported breaks during their diet.
Conclusions
Our preliminary data indicate that KDIs may be safe, feasible and potentially beneficial for ADPKD patients, highlighting that prospective clinical trials are warranted to confirm these results in a controlled setting and elucidate the impact of KDIs specifically on kidney function and cyst progression.
Food Restriction Ameliorates the Development of Polycystic Kidney Disease
Source: Journal of the American Society of Nephrology – https://journals.lww.com/jasn/pages/articleviewer.aspx?year=2016&issue=05000&article=00022&type=Fulltext
Date: 2016.05
Here, we show that food restriction (FR) effectively slows the course of the disease in mouse models of ADPKD. Mild to moderate (10%–40%) FR reduced cyst area, renal fibrosis, inflammation, and injury in a dose-dependent manner. Molecular and biochemical studies in these mice indicate that FR ameliorates ADPKD through a mechanism involving suppression of the mammalian target of the rapamycin pathway and activation of the liver kinase B1/AMP-activated protein kinase pathway. Our data suggest that dietary interventions such as FR, or treatment that mimics the effects of such interventions, may be potential and novel preventive and therapeutic options for patients with ADPKD.
Overweight and Obesity Are Predictors of Progression in Early Autosomal Dominant Polycystic Kidney Disease
Source: Journal of the American Society of Nephrology – https://journals.lww.com/jasn/pages/articleviewer.aspx?year=2018&issue=02000&article=00027&type=Fulltext
Date: 2018.02

Ketosis Ameliorates Renal Cyst Growth in Polycystic Kidney Disease
Source: Clinical and Translational Report https://doi.org/10.1016/j.cmet.2019.09.012
Date: 2019.12.03
Ketosis prevents or reverses PKD in animal models•
Dietary changes that induce ketosis prevent PKD•
Oral β-hydroxybutyrate supplementation alone inhibits PKD progression•
Cystic cells are metabolically inflexible, which can be exploited for therapy
Mild reduction in food intake was recently shown to slow polycystic kidney disease (PKD) progression in mouse models, but whether the effect was due to solely reduced calories or some other aspect of the diet has been unclear. We now show that the benefit is due to the induction of ketosis. Time-restricted feeding, without caloric reduction, strongly inhibits mTOR signaling, proliferation, and fibrosis in the affected kidneys in a PKD rat model. A ketogenic diet had a similar effect and led to regression of renal cystic burden. Acute fasting in rat, mouse, and feline models of PKD results in rapid reduction of cyst volume, while oral administration of the ketone β-hydroxybutyrate (BHB) in rats strongly inhibits PKD progression. These results suggest that cystic cells in PKD are metabolically inflexible, which could be exploited by dietary interventions or supplementation with BHB, representing a new therapeutic avenue to treat PKD.

Dietary Interventions in Autosomal Dominant Polycystic Kidney Disease
Source: Advances in Nutrition https://academic.oup.com/advances/article/13/2/652/6424888
Date: 2021.11.10
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by the progressive growth of renal cysts, leading to the loss of functional nephrons. Recommendations for individuals with ADPKD to maintain a healthy diet and lifestyle are largely similar to those for the general population. However, recent evidence from preclinical models suggests that more tightly specified dietary regimens, including caloric restriction, intermittent fasting, and ketogenic diets, hold promise to slow disease progression, and the results of ongoing human clinical trials are eagerly awaited. These dietary interventions directly influence nutrient signaling and substrate availability in the cystic kidney, while also conferring systemic metabolic benefits. The present review focuses on the importance of local and systemic metabolism in ADPKD and summarizes current evidence for dietary interventions to slow disease progression and improve quality of life.
Very Low-Calorie Ketogenic Diet: A Safe and Effective Tool for Weight Loss in Patients with Obesity and Mild Kidney Failure
Source: Nutrients https://www.mdpi.com/2072-6643/12/2/333
Date: 2020.01.27
Very low-calorie ketogenic diets (VLCKD) are an effective and increasingly used tool for weight loss. Traditionally considered high protein, ketogenic diets are often looked at with concern by clinicians due to the potential harm they pose to kidney function. We herein evaluated the efficacy and safety of a VLCKD in patients with obesity and mild kidney failure. A prospective observational real-life study was conducted on ninety-two patients following a VLCKD for approximately 3 months. Thirty-eight had mild kidney failure and fifty-four had no renal condition and were therefore designated as control. Anthropometric parameters, bioelectrical impedance and biochemistry data were collected before and at the end of the dietary intervention. The average weight loss was nearly 20% of initial weight, with a significant reduction in fat mass. We report an improvement of metabolic parameters and no clinically relevant variation regarding liver and kidney function. Upon stratification based on kidney function, no differences in the efficacy and safety outcomes were found. Interestingly, 27.7% of patients with mild renal failure reported normalization of glomerular filtrate after dietary intervention. We conclude that, when conducted under the supervision of healthcare professionals, a VLCKD is an effective and safe treatment for weight loss in patients with obesity, including those affected by mild kidney failure.
Intermittent Fasting: Physiological Implications on Outcomes in Mice and Men
Source: Journal of American Psychology Society https://journals.physiology.org/doi/full/10.1152/physiol.00030.2019
Date: 2020.04.15
Intermittent fasting (IF) is a widely practiced dietary method that encompasses periodic restriction of food consumption. Due to its protective benefits against metabolic diseases, aging, and cardiovascular and neurodegenerative diseases, IF continues to gain attention as a preventative and therapeutic intervention to counteract these chronic diseases. Although numerous animal studies have reported positive health benefits of IF, its feasibility and efficacy in clinical settings remain controversial. Importantly, since dietary interventions such as IF have systemic effects, thoroughly investigating the tissue-specific changes in animal models is crucial to identify IF’s mechanism and evaluate its potential adverse effects in humans. As such, we will review and compare the outcomes and underlying mechanisms of IF in both animal and human studies. Moreover, the limitations of IF and inconsistencies between preclinical and clinical studies will be discussed to provide insight into the gaps between translating research from bench to bedside.
Ketogenic Dietary Interventions in Autosomal Dominant Polycystic Kidney Disease (ADPKD) (Keto-ADPKD)
Source: University of Cologne https://clinicaltrials.gov/ct2/show/NCT04680780
Date: 2020.12.23, updated 2022.08.24
A mild reduction in food intake significantly inhibits renal cyst growth in mouse models of ADPKD. The underlying mechanism was unknown at the time. Recently published data show that the beneficial effect is not due to caloric restriction per se but due to the induction of the state of ketosis. Dietary interventions leading to ketosis profoundly inhibited renal cyst growth in rodent models of PKD. In addition, acute fasting led to rapid regression of renal cystic burden in mouse, rat and feline models of PKD. Due to these compelling effects in a multitude of PKD animal models, and due to the fact that well-established dietary interventions have a tremendous translational potential, KETO-ADPKD will test such interventions regimens in ADPKD patients.
Ren.Nu, a Dietary Program for Individuals with Autosomal-Dominant Polycystic Kidney Disease Implementing a Sustainable, Plant-Focused, Kidney-Safe, Ketogenic Approach with Avoidance of Renal Stressors
Source: Kidney Dial. https://www.mdpi.com/2673-8236/2/2/20
Date: 2022.04.13
Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited cause of renal failure and has limited pharmacological treatment options. Disease progression is relentless, and regression is not a known feature of ADPKD even with pharmacological intervention. Recent research has uncovered underlying pathogenic mechanisms that may be amenable to dietary interventions. Cyst cells in ADPKD are thought to depend on glucose for energy and are unable to metabolize fatty acids and ketones. High-carbohydrate diets and lifestyles leading to hyperglycemia appear to worsen progression of ADPKD. Additionally, renal stressors such as oxalate, phosphate and uric acid, that lead to renal tubular micro-crystal burden appear to accelerate disease progression. Based on these research findings, we have created a remote, dietitian-supervised training program to teach individuals with ADPKD the implementation of dietary and lifestyle changes to avoid factors that may worsen disease progression. Using web-based platforms, digital tools, one-on-one remote meetings, and video group meetings, participants learn to implement a plant-focused ketogenic diet that avoids renal stressors, the science behind these changes, how to self-measure health parameters, and track nutrient intake. Dietary changes are supplemented with a medical food containing the ketone beta-hydroxybutyrate and alkaline citrate, and mindfulness exercises. Here, we report the first experience with this program from a beta test with approximately 24 participants. Most participants completed the program and reported improvements in their health and well-being including pain levels, weight loss, hypertension, and eGFR. Adherence to the program was very high and the feasibility of the dietary and lifestyle changes was rated highly. The Ren.Nu program is now publicly available to individuals with ADPKD.
RESET-PKD: A pilot trial on short-term ketogenic interventions in autosomal dominant polycystic kidney disease
Source: Oxford Academic Nephrology Dialysis Transplantation https://academic.oup.com/ndt/advance-article-abstract/doi/10.1093/ndt/gfac311/6845752?login=false
Date: 2022.11.24

ABSTRACT
Background
Ketogenic dietary interventions (KDI) have been shown to be effective in animal models of polycystic kidney disease (PKD), but data from clinical trials are lacking.
Methods
Ten autosomal dominant PKD (ADPKD) patients with rapid disease progression were enrolled at visit V1 and initially maintained a carbohydrate-rich diet. At V2, patients entered one of the two KDI arms: a 3-day water fast (WF) or a 14-day ketogenic diet (KD). At V3, they resumed their normal diet for 3–6 weeks until V4. At each visit, magnetic resonance imaging kidney and liver volumetry was performed. Ketone bodies were evaluated to assess metabolic efficacy and questionnaires were used to determine feasibility.
Results
All participants [KD n = 5, WF n = 5; age 39.8 ± 11.6 years; estimated glomerular filtration rate 82 ± 23.5 mL/min/1.73 m2; total kidney volume (TKV) 2224 ± 1156 mL] were classified as Mayo Class 1C–1E. Acetone levels in breath and beta-hydroxybutyrate (BHB) blood levels increased in both study arms (V1 to V2 average acetone: 2.7 ± 1.2 p.p.m., V2 to V3: 22.8 ± 11.9 p.p.m., P = .0006; V1 to V2 average BHB: 0.22 ± 0.08 mmol/L, V2 to V3: 1.88 ± 0.93 mmol/L, P = .0008). Nine of 10 patients reached a ketogenic state and 9/10 evaluated KDIs as feasible. TKV did not change during this trial. However, we found a significant impact on total liver volume (ΔTLV V2 to V3: −7.7%, P = .01), mediated by changes in its non-cystic fraction.
Conclusions
RESET-PKD demonstrates that short-term KDIs potently induce ketogenesis and are feasible for ADPKD patients in daily life. While TLV quickly changed upon the onset of ketogenesis, changes in TKV may require longer-term interventions.
Preliminary Results Study: Influence of 3 months Keto Diet on kidney size and GFR
Source: https://portal.uni-koeln.de/en/universitaet/aktuell/press-releases/single-news/polycystic-kidney-disease-study-explores-diet-as-a-key-factor-in-kidney-disease (not working at the moment)
Alternative source: https://www.youtube.com/live/i9vjwns6aHY?feature=share&t=2393
Date: 2022

Kidney Size decrease on keto diet (green line)

GFR improved for Keto Diet Group (green line)

Metformin improves relevant disease parameters in an autosomal dominant polycystic kidney disease mouse model
Source: https://pubmed.ncbi.nlm.nih.gov/34806449/
Date: Nov 2022
Primary results of the randomized trial of metformin administration in polycystic kidney disease (TAME PKD)
Source: https://www.kidney-international.org/article/S0085-2538(21)00601-3/fulltext
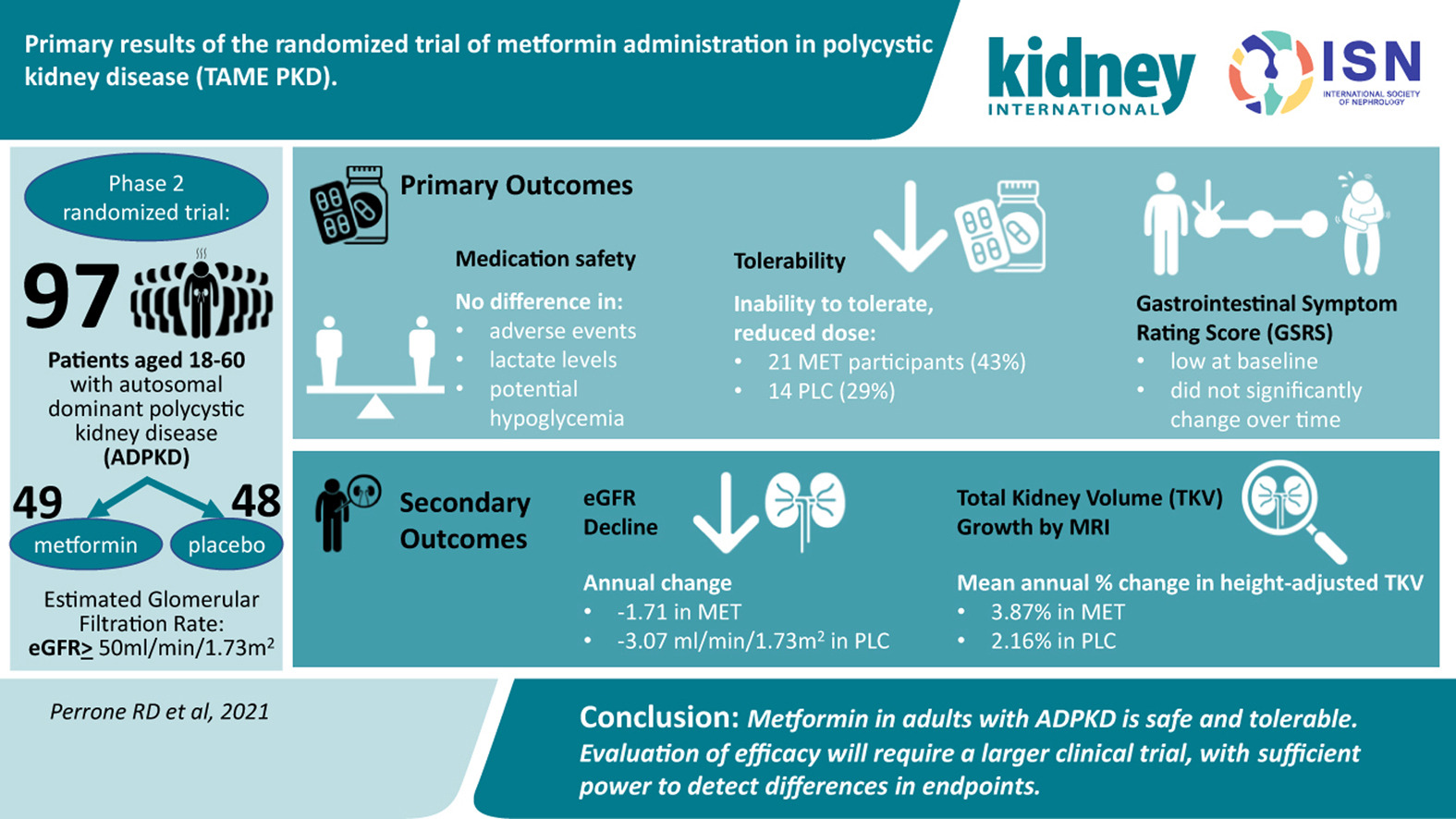
Watch for lactic acidosis + eGFR. Do not take metformin under 30
How you can contact me
go@startevo.com